
Myocardial Infarction Clinical Trial Pipeline Appears Robust With 45+ Key Pharma Companies Actively Working in the Therapeutics Segment | DelveInsight
The myocardial infarction market is driven by the growing prevalence of cardiovascular diseases and advancements in diagnostics and treatments. Key factors include an aging population, lifestyle-related risks like obesity and diabetes, and rising hypertension cases. Innovations in drugs and minimally invasive procedures, along with improved healthcare infrastructure, are enhancing patient outcomes. These trends underscore the market's significant growth potential.
/EIN News/ -- New York, USA, Jan. 13, 2025 (GLOBE NEWSWIRE) -- Myocardial Infarction Clinical Trial Pipeline Appears Robust With 45+ Key Pharma Companies Actively Working in the Therapeutics Segment | DelveInsight
The myocardial infarction market is driven by the growing prevalence of cardiovascular diseases and advancements in diagnostics and treatments. Key factors include an aging population, lifestyle-related risks like obesity and diabetes, and rising hypertension cases. Innovations in drugs and minimally invasive procedures, along with improved healthcare infrastructure, are enhancing patient outcomes. These trends underscore the market's significant growth potential.
DelveInsight’s 'Myocardial Infarction Pipeline Insight 2025' report provides comprehensive global coverage of pipeline myocardial infarction therapies in various stages of clinical development, major pharmaceutical companies are working to advance the pipeline space and future growth potential of the myocardial infarction pipeline domain.
Key Takeaways from the Myocardial Infarction Pipeline Report
- DelveInsight’s myocardial infarction pipeline report depicts a robust space with 45+ active players working to develop 50+ pipeline myocardial infarction drugs.
- Key myocardial infarction companies such as Kancera, Taiwan Bio Therapeutics, Translational Sciences, RION, Mesoblast, Faraday Pharmaceuticals, Idorsia Pharmaceuticals, Celecor Therapeutics, ResoTher Pharma, BioCardia, TRPHARM, Boehringer Ingelheim, and others are evaluating new myocardial infarction drugs to improve the treatment landscape.
- Promising pipeline myocardial infarction therapies such as KAND567, TWB201, TS 23, Purified exosome product, MPC-25-IC, FDY-5301, Selatogrel, Zalunfiban, RTP026, CardiALLO cell therapy, Goflikicept, BI765845, and others are under different phases of myocardial infarction clinical trials.
- In July 2024, CellProthera announced the commencement of the ‘PERFECT’ study, a long-term follow-up observational study of the patients involved in the recent Phase I/IIb EXCELLENT trial, following success with patients having received ProtheraCytes after severe heart attack (acute myocardial infarction) in the initial trial.
- In June 2024, Faraday Pharmaceuticals announced the completion of enrollment in its ongoing pivotal Phase III Iocyte AMI-3 trial of FDY-5301.
- In February 2024, Viatris announced they had entered into agreements for a significant global research and development collaboration under which Viatris would receive exclusive global development and commercialization rights to two Phase III assets, as well as the potential to add additional innovative assets in the future. The collaboration includes selatogrel, a potential life-saving self-administered medicine for patients with a history of acute myocardial infarction (AMI), or heart attack.
- In February 2024, Global Biotechnology announced top-line results from the Phase III AEGIS-II trial evaluating the efficacy and safety of CSL112 (apolipoprotein A-I [human]) compared to placebo in reducing the risk of major adverse cardiovascular events (MACE) in patients following an acute myocardial infarction (AMI).
- In January 2024, Faraday Pharmaceuticals announced its agreement with the Food and Drug Administration (FDA) on a Special Protocol Assessment (SPA) amendment, enabling the company to expedite its planned Phase III study interim analysis.
Request a sample and discover the recent advances in myocardial infarction drugs @ Myocardial Infarction Pipeline Report
The myocardial infarction pipeline report provides detailed profiles of pipeline assets, a comparative analysis of clinical and non-clinical stage myocardial infarction drugs, inactive and dormant assets, a comprehensive assessment of driving and restraining factors, and an assessment of opportunities and risks in the myocardial infarction clinical trial landscape.
Myocardial Infarction Overview
Myocardial infarction refers to the death of heart muscle tissue due to ischemia. The most common cause is coronary artery disease. Type 1 MI arises from the rupture of an unstable plaque, leading to coronary artery blockage. Type 2 MI occurs when there is an imbalance between oxygen supply and demand, such as during systemic hypotension or vasospasm. Clinically, myocardial infarction presents as acute coronary syndrome (ACS), a potentially life-threatening condition.
Myocardial infarction results from an imbalance between oxygen supply and demand. While significant atherosclerosis causing ≥75% narrowing of a coronary artery may not affect blood flow at rest, increased myocardial demand during activities like exercise or tachyarrhythmias can lead to ischemia and angina pectoris. In most cases, MI is caused by coronary atherosclerosis complicated by thrombosis. Plaque rupture is the primary trigger for thrombosis, exposing the necrotic core of the plaque to the bloodstream, which induces a strong clotting response. Although most patients with MI have obstructive coronary disease, plaque rupture, and ulceration can occasionally occur without a visible obstructive lesion. Rare conditions, such as coronary embolism from endocarditis, prosthetic valve thrombosis, coronary artery dissection, or autoimmune/infectious arthritis, can also lead to non-atherosclerotic MI.
Pathophysiologic factors contributing to oxygen imbalance play a key role in MI. Coronary vasospasm and endothelial dysfunction may restrict blood supply, even without significant blockages, while severe anemia can impair oxygen delivery to the heart. Conversely, conditions that elevate myocardial oxygen demand, such as thyrotoxicosis, aortic stenosis, or cocaine use, may trigger MI even with relatively minor reductions in supply.
Diagnosis is based on clinical symptoms, ECG changes, and elevated cardiac biomarkers. Definitive confirmation requires cardiac catheterization, which is both diagnostic and therapeutic. Patients suspected of ACS should undergo emergency revascularization. Treatment involves anticoagulants, antiplatelet agents, statins, and other supportive therapies. Secondary prevention includes dual antiplatelet therapy, beta-blockers and/or ACE inhibitors, statins, and addressing modifiable risk factors.
Find out more about myocardial infarction drugs @ Myocardial Infarction Analysis
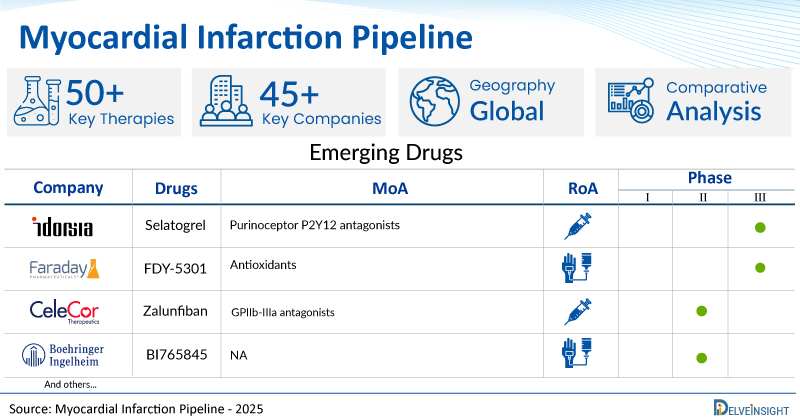
A snapshot of the Pipeline Myocardial Infarction Drugs mentioned in the report:
| Drugs | Company | Phase | MoA | RoA |
| Selatogrel | Idorsia Pharmaceuticals | III | Purinoceptor P2Y12 antagonists | Subcutaneous |
| FDY-5301 | Faraday Pharmaceuticals | III | Antioxidants | Intravenous |
| Zalunfiban | Celecor Therapeutics | II | GPIIb-IIIa antagonists | Subcutaneous |
| BI765845 | Boehringer Ingelheim | II | NA | Intravenous |
| RTP-026 | ResoTher Pharma | II | Formyl peptide receptor modulators | Intravenous |
Learn more about the emerging myocardial infarction therapies @ Myocardial Infarction Clinical Trials
Myocardial Infarction Therapeutics Assessment
The myocardial infarction pipeline report proffers an integral view of the emerging myocardial infarction therapies segmented by stage, product type, molecule type, route of administration, and mechanism of action.
Scope of the Myocardial Infarction Pipeline Report
- Coverage: Global
- Therapeutic Assessment By Product Type: Mono, Combination, Mono/Combination
- Therapeutic Assessment By Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III
- Therapeutics Assessment By Route of Administration: Intravenous, Subcutaneous, Oral, Intramuscular
- Therapeutics Assessment By Molecule Type: Monoclonal antibody, Small molecule, Peptide
- Therapeutics Assessment By Mechanism of Action: Sodium-glucose transporter 2 inhibitors, Chemokine CXCL13 inhibitors, Antiplasmin inhibitors, Fibrinolytic agents, Cell replacements, Antioxidants, Purinoceptor P2Y12 antagonists, GPIIb-IIIa antagonists
- Key Myocardial Infarction Companies: Kancera, Taiwan Bio Therapeutics, Translational Sciences, RION, Mesoblast, Faraday Pharmaceuticals, Idorsia Pharmaceuticals, Celecor Therapeutics, ResoTher Pharma, BioCardia, TRPHARM, Boehringer Ingelheim, and others.
- Key Myocardial Infarction Pipeline Therapies: KAND567, TWB201, TS 23, Purified exosome product, MPC-25-IC, FDY-5301, Selatogrel, Zalunfiban, RTP026, CardiALLO cell therapy, Goflikicept, BI765845, and others.
Dive deep into rich insights for new myocardial infarction treatments, visit @ Myocardial Infarction Drugs
Table of Contents
| 1. | Myocardial Infarction Pipeline Report Introduction |
| 2. | Myocardial Infarction Pipeline Report Executive Summary |
| 3. | Myocardial Infarction Pipeline: Overview |
| 4. | Analytical Perspective In-depth Commercial Assessment |
| 5. | Myocardial Infarction Clinical Trial Therapeutics |
| 6. | Myocardial Infarction Pipeline: Late-Stage Products (Pre-registration) |
| 7. | Myocardial Infarction Pipeline: Late-Stage Products (Phase III) |
| 8. | Myocardial Infarction Pipeline: Mid-Stage Products (Phase II) |
| 9. | Myocardial Infarction Pipeline: Early-Stage Products (Phase I) |
| 10. | Myocardial Infarction Pipeline Therapeutics Assessment |
| 11. | Inactive Products in the Myocardial Infarction Pipeline |
| 12. | Company-University Collaborations (Licensing/Partnering) Analysis |
| 13. | Key Companies |
| 14. | Key Products in the Myocardial Infarction Pipeline |
| 15. | Unmet Needs |
| 16. | Market Drivers and Barriers |
| 17. | Future Perspectives and Conclusion |
| 18. | Analyst Views |
| 19. | Appendix |
For further information on the myocardial infarction pipeline therapeutics, reach out @ Myocardial Infarction Therapeutics
Related Reports
Myocardial Infarction Epidemiology Forecast
Myocardial Infarction Epidemiology Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted myocardial infarction epidemiology in the 7MM, i.e., the United States, EU5 (Germany, Spain, Italy, France, and the United Kingdom), and Japan.
Myocardial Infarction Market Insights, Epidemiology, and Market Forecast – 2034 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, market share of the individual therapies, and key myocardial infarction companies including AstraZeneca, Boehringer Ingelheim and Eli Lilly and Company, Amgen, Novartis, Idorsia Pharmaceuticals, Faraday Pharmaceuticals, CSL Behring, Immediate Therapeutics, Mitsubishi Chemical Group, Kancera, Bayer, Recardio, Mesoblast, among others.
Acute Myocardial Infarction Market
Acute Myocardial Infarction Market Insights, Epidemiology, and Market Forecast – 2034 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key acute myocardial infarction companies including Boehringer Ingelheim, Eli Lilly and Company, Idorsia Pharmaceuticals, Recardio, Janssen Pharmaceutical, Bristol Myers Squibb, AstraZeneca, Faraday Pharmaceuticals, CSL Behring, Amgen, among others.
Myocarditis Market Insights, Epidemiology, and Market Forecast – 2034 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key myocarditis companies including Cardiol Therapeutics, Cardiol Therapeutics, Apitope, among others.
Myocarditis Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key myocarditis companies, including Cantargia AB, Cardiol Therapeutics, Apitope, among others.
DelveInsight’s Pharma Competitive Intelligence Service: Through its CI solutions, DelveInsight provides its clients with real-time and actionable intelligence on their competitors and markets of interest to keep them stay ahead of the competition by providing insights into the latest therapeutic area-specific/indication-specific market trends, in emerging drugs, and competitive strategies. These services are tailored to the specific needs of each client and are delivered through a combination of reports, dashboards, and interactive presentations, enabling clients to make informed decisions, mitigate risks, and identify opportunities for growth and expansion.
Other Business Consulting Services
Healthcare Conference Coverage
Discover how a mid-pharma client gained a level of confidence in their soon-to-be partner for manufacturing their therapeutics by downloading our Due Diligence Case Study
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences.
Connect with us at LinkedIn

Contact Us
Shruti Thakur
info@delveinsight.com
+14699457679
www.delveinsight.com
Distribution channels: Banking, Finance & Investment Industry, Healthcare & Pharmaceuticals Industry, Media, Advertising & PR, Science ...
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release

